The Interdepartmental Equipment Facility
EA - Elemental Analysis of C, H, N, S and O.

The Flash EA 1112 Elemental Analyzer is used for determination of total carbon (C), hydrogen (H), nitrogen (N), sulfur (S) and oxygen (O).
The instrument is used for analyses of a wide range of organic and inorganic samples such as soils and sediments, plant tissue, pharmaceuticals, fuels, polymers, and many others. The chemical characterization of organic compounds plays an important role in all processes of synthesis and analysis of organic compounds for both research and quality control purposes. In many cases it is possible to achieve a complete characterization of a compound in a single analysis run.
Sample Preparation. The analyzed sample is expected to represent the tested material faithfully. The size of the sample, its water content the degree of milling and homogenization should be chosen and controlled to that end.
Organic compounds including pharmaceutical products are frequently used without any pre-treatment, if known to be fully homogeneous. Soils, grain samples and other plant material are dried first and then ground with a mill, down to a particle size of 0.2 to 0.5 mm in diameter. Protein or fat samples are frozen with liquid nitrogen before they are ground. The material becomes brittle allowing easier powder formation. Plastics, polymers, paper, rubber etc., are similarly ground using liquid nitrogen.
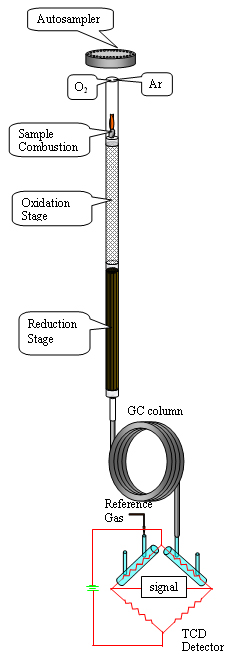
Dynamic Flash Combustion. A small amount of the sample to be analyzed, typically 2-3 mg, is now wrapped in a small tin-foil capsule and weighed with a microbalance to the nearest 1 microgram. Samples are placed in the MAS 200 Autosampler drum, where they are purged with helium, which serves as the carrier gas for the whole process. Each sample, in turn, is dropped into the combustion reactor: a vertical quartz tube, maintained at 1020oC and packed with two separate layers of catalysts which facilitate the next stages of the process.
Oxidation Stage. As a sample is dropped into the furnace, the helium stream is temporarily enriched with pure oxygen. The sample and its container melt and the tin promotes a violent flash combustion, elevating the temperature further to well above 1800oC. The sample is vaporized and combusted to form CO2, H2O, SO2 and nitrogen oxides. The cloud of hot gases is now pushed down the reactor by the carrier gas, passing over a layer of a copper oxide catalyst which promotes complete oxidation of all elements.
Reduction Stage. The mixture of combustion gases is next pushed through a layer of closely packed pure-copper wires which removes the excess of oxygen gas and reduces the different nitrogen oxides to elemental nitrogen. Also, SO3 is reduced at this stage to SO2.
Chromatographic Separation. The gas mixture is now swept by the carrier gas to the chromatographic column where the individual components are separated as Nitrogen, Carbon dioxide, Water and Sulfur dioxide.
A Thermal Conductivity Detector (TCD), connected to the carrier-gas pathway after the column, detects the different gases as they come out of the column with different retention times.
The TCD is a universal detector responding to most compounds including gases such as oxygen, nitrogen, and carbon dioxide. The TCD consists of sample and reference flow cells drilled into a metal block. Tungsten-Rhenium filaments which exhibit temperature-dependent electrical resistance are suspended in each flow cell.
The electrical current flowing through the filaments heats them up and the heat is dissipated by the carrier gas flow around the filaments. When a analyte peak is eluted from the column, the different thermal conductivity of the carrier-analyte mixture causes a change in the filament temperature in the sample cell while the reference temperature remains constant. The change in filament resistance caused by an analyte passing through the sample flow cell is measured with a Wheatstone bridge circuit.
The TCD signal is presented as a function of time in a chromatogram. The signal is digitized and the area under each peak is integrated by the computer, and compared with calibration curves obtained for the different elements with standard compounds.
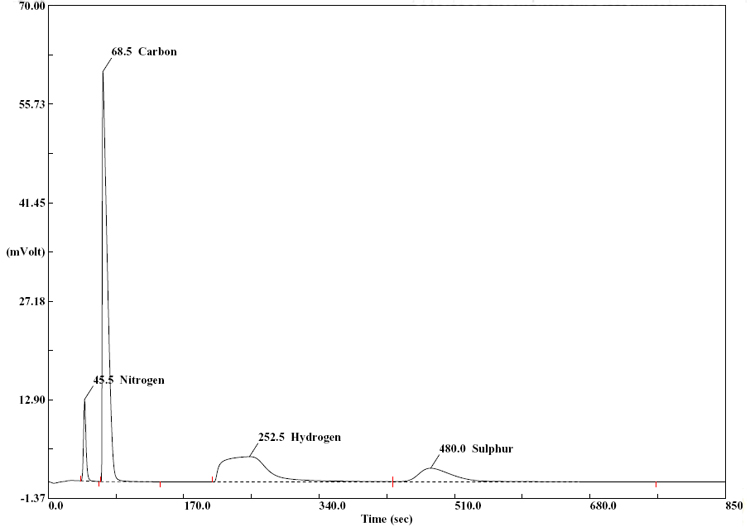
A complete report is automatically generated by the Eager 300 software and displayed at the end of each analytical cycle. The previously recorded weight of a sample is now used to calculate the concentrations of the four elements - C, H, N, and S as "% by weight".
Calibration. Pure organic compounds are used to calibrate and validate the system. Several standards are available, covering a wide range of element compositions. The chosenstandards should be as similar as possible to the analyzed samples.
"Memory Effect" is usually minimal but should be checked if samples with a low content of an element are to be analyzed right after samples with high proportions of that element. An empty tin capsule, serving as a blank, can be used to estimate the extent of the memory effect.