The Interdepartmental Equipment Facility
FLA-5000 Imaging System
for Fluorescent and Radioactive samples

The FLA-5000 is a laser-based imaging system that allows scanning of fluorescent as well as radiolabeled samples. The system is especially useful for two-dimensional protein electrophoresis and for array analysis in genomics, as it allows scanning of samples as large as 40 x 46 cm.
Resolution. The scanned area is divided into pixels whose size can be either 25, 50, 100 or 200 µm. Scanning at the highest resolution of 25 µm per pixel, across a lane containing protein bands about 1mm apart, should yield around 40 pixels per band. In many cases a lower resolution of 100 µm is enough, as scanning large areas at high resolution results in huge data files.

Sample holding Stages. The FLA-5000 is equipped with three sample-holding stages. The Glass Stage can accommodate large polyacrylamide gels. The IP Stage is a full metal stage for radioactivity Imaging Plates (or IPs). The stage is magnetized and IPs attach to it from below - face down. The Microplate holder Stage has six slots for up to six microtiter plates. Stages are inserted as drawers at the top of the Instrument, just above the optics. Note that the laser beams illuminate the samples from below.

Lasers and Filters.
The FLA-5000 imager is equipped with three lasers, blue, green and red, which are used to excite fluorescence in the scanned samples. Light emitted by the samples is filtered through an appropriate 'emission-filter' which blocks stray scattered light, permitting only fluorescence at wavelengths well above the laser line to pass through to the PMT detector.
The emission filters are placed in the filter tray which can be found behind the small door seen on the front of the instrument. The software can be used to control repetitive scanning of a sample by different combinations of lasers and emission filters. The following table presents the combination of lasers and filters used for several applications.
| Applications & dyes | Laser to use | Wavelength (nm) | Filter to use |
|---|---|---|---|
| SYPRO Orange, SYBR Green I, FITC |
SHG blue laser, 2mW | 473 | Y510 |
| Cy3, Rhodamine, SYPRO Red |
SHG green laser, 5.5 mW | 532 | 0575 |
| Cy5 | LD red laser, 20 mW |
635 | R665 |
| Imaging Plates for Radioisotopes | LD red laser, 20 mW | 635 | B390 |
SHG - Second Harmonic Generation Laser. LD - laser Diode
Imaging Plates
Imaging of gels labeled with radioactive tracers is accomplished by placing the gel together with an Imaging Plate in a light-tight cassette for several hours.
The Imaging Plates (IPs) that we use consist of rigid plastic sheets uniformly coated with phosphor. The coating consists of small crystals (~ 5 µm) of barium fluorobromide doped with a trace amount of bivalent europium, which serves as energy trap and luminescence center. An additional thin coating protects the phosphor layer against physical and chemical damages.
An electron excited by beta or x-ray radiation, coming from the gel, is trapped in the crystal in a metastable state. After the IP has been exposed to the radioactive sample for an appropriate period, it is taken out of the light-tight cassette and inserted into the FLA-5000 reader, where it is scanned with the red laser beam. Depending on the application, reading resolution can vary between 5 to 40 pixels/mm, which means pixel size of 200 µm to 25 µm respectively.
The red laser photons kick the trapped electrons out of their meta-stable energy wells into the crystal conduction band, where they wander around until they are trapped by a divalent Europium center. Here the extra energy is released as luminescence and the phosphor returns to its ground state.
The purple (400 nm) luminescence, released upon laser excitation, is focused by a light collection guide onto the photomultiplier tube (PMT). The voltage converted to a digital signal and registered as the light intensity for this specific pixel. At the end of the scanning process an image of the scanned gel is generated. The luminescence intensity at each pixel reflects the radiation intensity of a corresponding 'pixel' on the gel. The resultant image can be printed and the data can be further processed in a variety of ways.
Detectable Nuclides include among else 32P, 14C, 35S,125I, and 3H. Tritium requires a special Imaging Plate, as a significant portion of its weak beta particles fails to penetrate the protective layer covering the standard IPs.

The Eraser
The Imaging Plate cab be used again after erasing the residual latent image with uniformly irradiated visible light. In practice this is done by a special 'Eraser', where the IP is exposed to a battery of fluorescent lamps for a pre-programmed period of time, making it ready for the next imaging session. It is good practice to 'erase' the IP immediately before the next exposure, in order to minimize random noise due to stray ionizing radiation.
The IP compared to an X-ray Film
The differences between an imaging plate and an X-ray film are illustrated below. The figure compares detection by the two methods of radiation emitted a series of standards tagged with of 32P. The left ordinate is the radiation intensity recorded by an imaging plate and the right ordinate is the changes induced in an X-ray film by exposure to the same standards. The abscissa shows the radioactivity of the different 32P standards as measured with a liquid scintillation counter.
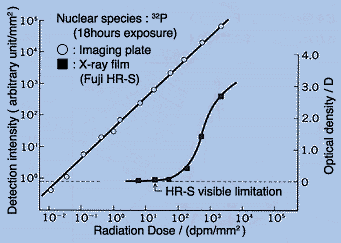
1. Higher sensitivity: by at least two orders of magnitude.
2. Wider dynamic range: four to five orders of magnitude compared to 1-2.
3. Better linearity. The response is linear with the dose over the entire range.
4. Digital output is directly available. Data processing is easy.
5. The accumulated background can be erased before use.
6. The IP plate can be reused.