The Interdepartmental Equipment Facility
Ion Chromatography - IC

The Dionex ICS 3000 Ion Chromatography System is used for separation and quantitative analysis of ions. The ions in the sample are separated by an ion exchange column and are measured using a coductivity detector.
Ion exchange columns retain analyte molecules based on ionic interactions. The stationary phase displays ionic functional groups that bind ions of the opposite charge.
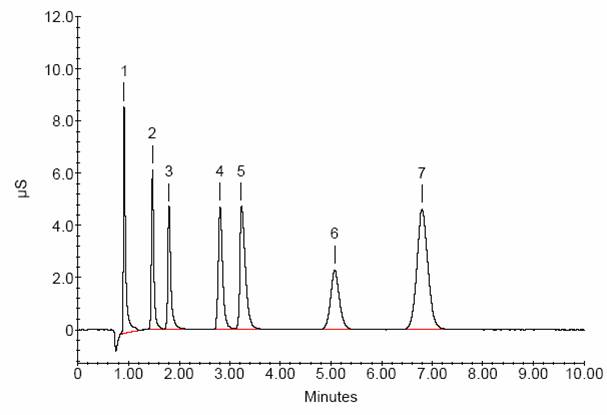
Ion exchange chromatography is further divided into cation and anion chromatography. Cation exchange columns have negatively charged functional groups and retain cations, while anion exchange columns retain anions, using positively charged functional groups. The chromatogram shown below illustrates sequential determination of seven common anions, including fluoride (1), chloride (2) ,nitrite (3), bromide (4), nitrate (5), phosphate (6), and sulfate (7).
Anion Analysis by Ion Chromatography
The Ion Chromatography Process

An aqueous sample is injected with a syringe, either manually or using an autosampler, into a sample loop of known volume. When a 'run' is initiated, the valve switches so as to include the sample loop in the eluent pathway. The eluent, a KOH solution driven by a high-pressure pump, carries the sample from the loop into the anion exchange column. Eluent and sample now flow through the column.
The column is packed with small particles of an anion exchange resin, with covalently bonded positively charged binding groups. The different anions in the sample are separated by the column according to their relative affinity for the positive binding sites on the anion exchanger.
The eluent anions, which keep on coming, compete with the sample anions for the positive binding-sites on the Ion-Exchanger, and gradually dislodge the sample-anions from their binding sites. The sample anions now wash down the column. Anions with higher affinity for the binding sites last longer, they elute later, and accordingly have longer 'retention-times'.
The Suppressor. The separated analytes next flow one by one into the Conductivity Suppressor Column. Its role is to reduce the background conductivity of the eluent to a minimum, and increase the coductivity of the analytes, and as a result increase the signal / noise ratio of the analyzed anions to a maximum.

The sample anions, accompanied by potassium counterions, flow in the suppressor column inside a hollow fiber made of a cation exchange membrane. The potassium cations driven away by an electric field, are replaced by protons, which are continuously formed by electrolysis of water. The eluent conductivity is suppressed because the incoming protons combine with the eluent hydroxide-ions to form the poorly conducting water. In addition, anylate conductivity is enhanced because the analyte anions are now balanced by highly mobile protons.
The concentrations of the separated anions are measured by a conductivity detector. Anions are identified by their retention time, which is determined by injecting separate standards for the different anions. The electronic signal is processed by the computer and quantitation is achieved by measurement of peak areas.