The Interdepartmental Equipment Facility
ICP - Atomic Emission Spectrometer
The ICP/AES is usually referred to as ICP in short. The full acronym stands for Inductively Coupled Plasma / Atomic Emission < b>Spectrometer.
We use 'ARCOS' ICP Spectrometers from Spectro GMBH, Kleve, Germany to detect, identify and measure the concentrations of metals (as well as most other elements), in samples from a variety of sources. ICPs are widely used for environmental and geological applications, as well as for agricultural, biological and pharmaceutical samples, and for tests of food and drinking water quality.
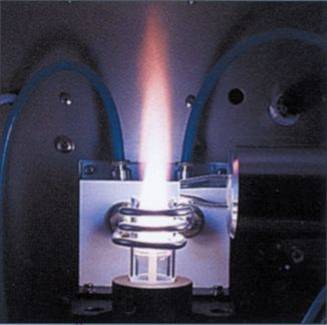
Description of the process. A liquid sample is sprayed into an argon plasma torch burning at 6000-10000oK. The injected sample is quickly dehydrated and its solutes first melt and then evaporate by the heat. Molecules break down into free atoms and a large proportion of these atoms are also ionized.
Free atoms and ions generated in the plasma appear to glow in this inferno: electrons in their outer shells hop to higher orbits, and as they fall back to the ground state light is emitted. This glow is a unique collection of discrete wavelengths characteristic of the different elements in the sample.
Radial vs. Axial Plasma The Two ARCOS Spectrometers look alike, but actually offer two different modes of operation. In the traditional mode the torch is "radial" or vertical, and radiation is detected at a 90-degree angle to the torch axis. The other spectrometer has an "axial" or horizontal plasma, where light emission is detected along the torch axis.
The radial plasma is more robust. It is less effected by high salt or acid concentrations and is relatively free from interferences - for a considerable array of elements. The axial plasma on the other hand, facilitates a much more efficient collection of analyte light emission and improves the analytical performance, by reducing the limit of detection significantly for most elements.
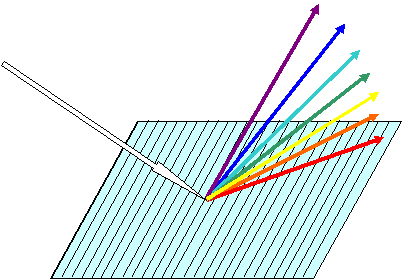
Light Dispersion and Detection. The multi-colored light beam emitted by the torch is dispersed by a grating to generate a rainbow of lines at different wavelengths between 130-770 nm. Each line, originally emitted by a single element, is now aimed at a specific detector placed in a pre-determined location in a chain of linear CCD elements arranged in a circle. The CCD translates the radiation intensity into electric current of proportional intensity.
Calibration. The signal generated by the ICP spectrometer is linearly proportional to element concentration in solution, over 3-5 orders of magnitude. Calibration curves for the different elements are constructed by measuring multi-element standard solutions spanning the relevant concentration range.
Limit of Detection. Most elements are detected by the ICP at concentrations lower than 1 ppb (Table I). Limit-of-Detection values for the axial-ICP are significantly lower than those obtained with the radial-ICP.
Background Subtraction Background Subtraction is used to correct for changes in the 'baseline', i.e. non-specific, broadband increase or decrease of light emission. The correction is typically carried out by measuring the emission level on the two sides of the analyte peak, and subtracting their average from the peak value.
Matrix Matching Matrix composition strongly affects the analytical results. High acid or high matrix-element concentrations may either depress or enhance the analyte signal, compared to standard solutions typically prepared in 1% HNO3. This problem can be solved by preparing the standards in a matrix that matches the matrix of the samples.
Internal Standard. An internal standard is added to samples to correct for the loss of analytes during sample preparation, and for matrix effects on the analysis. Apparent changes in standard concentration are expected to portray parallel changes in analyte concentration. An ideal internal standard element should not be present in the samples. It should be compatible with the samples and matrix, and should not have spectral overlap with the analytes. Scandium (Sc) and Yttrium(Y) are commonly used as internal standards.
Spectral interferences Spectral interferences are due to overlapping between analyte lines and light emission from other sources. This can be a contribution from an interfering line of another element, which leads to overestimation of the analyte concentration. This type of interference is overcome by either calculating the contribution of the 'interferent' and subtracting it from the overall emission intensity, or simply by choosing another (undisturbed) line for the analyte - if such a line is available.
Another type of interference can be caused by nonspecific scattered light in the spectrometer or from the 'tail' of an intense peak resulting in increase in background around the analyte line. Simple subtraction of background adjacent to the analyte line can often correct for this type of spectral interference.

Sample Preparation. Most samples have to be prepared for analysis. The samples are digested, usually in strong mineral acids. Solid samples are solubilized and organic matter is 'mineralized' - i.e. hydrolized and oxidized exhaustively until it is converted to inorganic compounds. Sample digestion is frequently performed in a Microwave Digestion System. This can be regarded as a combination of a microwave oven and a pressure-cooker. The final product expected is a clear solution of inorganic minerals.
The efficiency of the digestion process is estimated by measuring the 'recovery' of each element in a sample 'spiked' with known amounts of the element. An internal standard is often added for similar purposes.
The Main advantages of ICP/AES
Wide dynamic range. The signal varies linearly with concentration over 3 to 5 orders of magnitude, compared with a single decade of linearity for an Atomic Absorption Spectrometer (AAS).
Low Detection Limits. The ICP is about 100 to 1000-fold more sensitive than flame-AAS. This is especially evident for "refractory" elements such as Ti, Zr, Al, Si, Mo etc.
Simultaneous Measurements. The ICP can measure simultaneously a large number of elements, while the AAS is practically limited to one element at a time.