The Interdepartmental Equipment Facility
Liquid Nitrogen - LN2
Liquid nitrogen or LN2 is pure nitrogen in the liquid state. It is a colorless clear liquid, with a density of 0.807 g/mL. LN2 is manufactured by fractional distillation of liquid air, and serves as coolant in a large variety of applications, notably storing and preserving biological samples. LN2 is a cryogenic fluid which can cause rapid freezing and considerable damage to living tissue. It must be handled with extreme care.

Why does LN2 look like it is boiling all the time?
At atmospheric pressure, liquid nitrogen boils at -196 C, and in practice we find it indeed boiling at all times. This is not surprising as the ambient temperature is some 220°C higher. This may be compared to putting a kettle with water at 20°C on a hot plate set to 240°C. The water will reach its boiling point at 100°C and stay there boiling. All heat flow from the hot plate will now be used solely for evaporation, and the temperature will stay at 100° as long as there is liquid water left in the kettle.
Similarly, liquid nitrogen will keep on boiling, giving of nitrogen gas, but the liquid and any samples suspended in it will stay at -196°C as long as there is liquid nitrogen left in the flask.
Safety Precautions
- 1. Do not seal liquid nitrogen in air-tight containers. Pressure will build up inside and cause the container to explode.
- 2. LN2 can inflict severe burns. Use protective clothing including goggles, gloves, work shoes and an apron.
- 3 . Use LN2 only in well ventilated areas. Evaporation of LN2 may reduce the oxygen levels to life threatening levels.
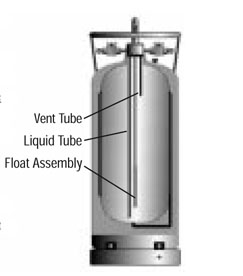
Dewars
Our LN2 is stored in 160L stainless steel, double-walled, evacuated Dewar containers. The vacuum between the two walls reduces heat transfer and minimizes loss of LN2 by evaporation. These dewars can hold their liquid nitrogen load for about three weeks. The float assembly illustrated in the diagram on the right serves as a dipstick, indicating the amount of liquid left in the cylinder.
The 160L containers are used to dispense LN2 into smaller dewars. This big container is built like a soda syphon bottle, with the liquid-drawing tube extending inside all the way down. The boil-off nitrogen gas accumulates in the head-space of the container and drives out liquid through the tube - once the liquid valve is manually opened.
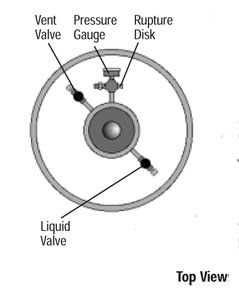
A pressure-release valve, set to ~ 1.5 bar, regulates the gas pressure in the head space, to facilitate gentle delivery of the liquid. Usually you can hear its silent hissing as it releases the extra gas pressure. A rupture disk is also installed to prevent the buildup of dangerous pressures in the event of a malfunction of the pressure release valve. The pressure gauge should indicate about 1.5 bar (or ~22 p.s.i) at all times.
The vent valve seen in the top view in the diagram on the right, is connected
to a vent tube which goes into the gas-containing headspace. It can be used to
release the gas pressure, if necessary, but in many cases it is used as a source of
pure and dry nitrogen gas for general laboratory use. The design of the 160L dewar
can actually be modified, by adding heating elements, to facilitate extensive
conversion of liquid into gas. A single 160 L cylinder of liquid is equivalent
to about 15 cylinders of compressed nitrogen gas.
Selected Physical Properties of Nitrogen
- * Molecular Weight : 28.01
- * Boiling Point : -195.8°C
- * Freezing Point : -210.0
- * Specific Gravity of Liquid, at 20°C and 1 atm : 0.808
- * Specific Gravity of gas ( with air=1) at 20°C and 1 atm : 0.967
- * Expansion Ratio of Liquid to Gas : 1 to 694