The Interdepartmental Equipment Facility

The Synergy-2 Multimode Microplate Reader
The BioTek Synergy 2 offers three modes of operation for 6- to 384-well plates:
1. Absorbance,
2. Luminescence,
3. Fluorescence.
Absorbance can be measured in the UV and Visible ranges, up to 4.0 OD, with a resolution of 0.001 OD (absorbance units). Any wavelength between 200 and 999 nm can be selected with 1 nm increments. A Xenon flash is used as light source and a monochromator serves for wavelength selection. Three basic modes of absorbance measurement can be selected: 1. Concentration measurements using calibration curves, 2. Kinetic measurements carried out by recording absorbance changes versus time and 3. Absorbance Spectra that are obtained by measuring absorbance as function of wavelength. The microplate reader is set up for temperature control and for shaking. A reading of a 96-well plate can be performed in as little as 11 seconds.
Luminescence between 300 and 700nm can be monitored as a function of time. The system can detect as little as 0.01 femtomole of ATP, and up to six orders of magnitude higher concentrations.
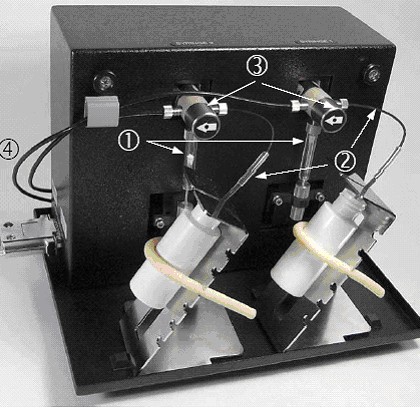
The instrument is equipped with two Reagent Dispensers. The dispensers
can be programmed to deliver two different reagents during the reaction time
course. A syringe (#1) draws the reagent solution from the vial (#2), and then
drives it through the switching valve (#3) towards the wellplate (#4).
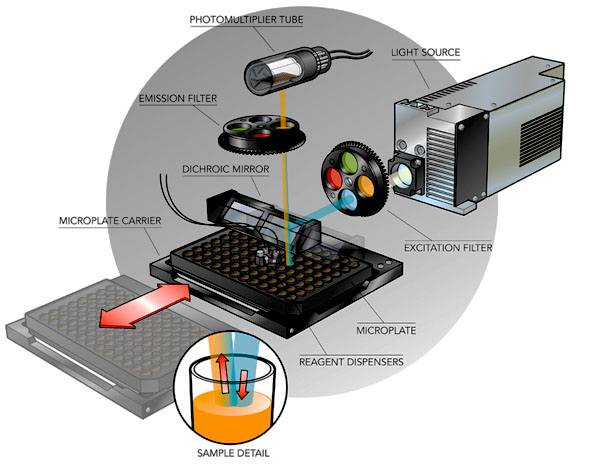
The fluorescence system has a tungsten-halogen lamp as light source, and uses filters and dichroic mirrors to select wavelengths for excitation and emission. Synergy-2 can detect 1pM of fluorescein, i.e. 0.2 femtomole / well in a 96-well plate. The dynamic range is > 6 decades. The excitation and emission pathways are each equipped with a four-position filter wheel - the filters are replaceable (see the scheme at the bottom of this page).
Table I shows our current collection of filters. If you plan to use the Synergy-2 for fluorescence measurements, you should first determine what wavelengths you want to use for the excitation and the emission, and then select the appropriate filters from table I. Call Israel Meir at 08-9489810 to set up the instrument for your experiment.
Table I - Optical Filters available for Fluorescence Measurements
| wavelength (nm) | bandwidth (nm) | color | Main Applications |
|---|---|---|---|
| 284 | 10 | uv | Tryptophan excitation |
| 340 | 30 | uv | NADH excitation and tryptophan emission |
| 360 | 40 | uv | MUB, caspace-3, europium chelate excitation |
| 380 | 20 | Fura-2 and EBFP excitation | |
| 420 | 50 | CFP excitation and Quanta-Blu emission | |
| 440 | 40 | NADH emission | |
| 460 | 40 | NanoOrange excitation and EBFP and MUB emission | |
| 485 | 20 | Fluorescein, EGFP excitation and CFP emission | |
| 500 | 27 | YFP excitation | |
| 508 | 20 | Fura-2 emission | |
| 528 | 20 | VIC excitation and Fluorescein and EGFP emission | |
| 540 | 25 | Alexa Fluor 546, CY3, and rhod2 excitation and EYFP emission | |
| 575 | 15 | ROX excitation and CY3 and 5-Tamra emission | |
| 590 | 20 | Alexa Fluor 594 and Texas Red excitation and Cell Titer Blue emission | |
| 600 | 40 | Alamar Blu, Amplex Red, RFP and porphyrin emission | |
| 645 | 40 | Texas Red and Propidium iodide emission |
Synergy 2 - Optical Scheme of Fluorescence Measurements