The Interdepartmental Equipment Facility
The β-Counter

Most of the radioisotopes used in our labs emit β particles, as they disintegrate spontaneously. A Liquid Scintillation counter such as the Packard 1900 TR was designed to detect and measure the intensity of β radiation.
β particles are very fast electrons, with kinetic energies in the keV and Mev ranges. Once launched, a β particle starts colliding with whatever stands in its way, transferring energy to the surroundings. Eventually it loses its speed and comes to a rest. With all that, β particles do not travel very far. Most are unable to penetrate a liquid scintillation vial. Β particles emitted by tritium cannot penetrate a sheet of paper. It takes a liquid scintillation counter to pick up such "soft" radiation.
The Liquid Scintillation Cocktail
In order to detect β particles, radioactive samples are immersed in a cocktail consisting of a fluorescent dye in a solvent. An emulsifier is also added to facilitate proper mixing of aqueous samples in the organic solvent. The scintillation cocktail converts the kinetic energy of the particles into visible light which can be detected by the photomultipliers of the scintillation counter.
A β particle released into such a cocktail bumps into myriad solvent molecules before coming to a stop. The excited solvent molecules pass this extra energy around and some of it is eventually trapped by fluorescent-dye molecules. The 'fluor' molecules cut short this process of energy transfer by emitting photons and returning to the ground state.
The energy of a single β particle is orders of magnitude higher than the energy of a visible photon. Hundreds and thousands of photons are released in a fraction of a second, following the launch of a single β particle into the scintillation cocktail.
The number of photons emitted is proportional to the energy of the particle (see the illustration below). The higher the speed of the β particle, the longer it travels, more solvent molecules are excited and more light is generated. While a tritium beta particle, with maximum energy of 18.6 keV generates about 30 photons, a C-14 beta particle with 156 keV can generate 250 photons and a P-32 beta particle, can use its 1,71 MeV to produce as many as 3300 light photons. Note however that the fluorescence yield is quite low in all cases: only about 1% of the β energy is converted into light.

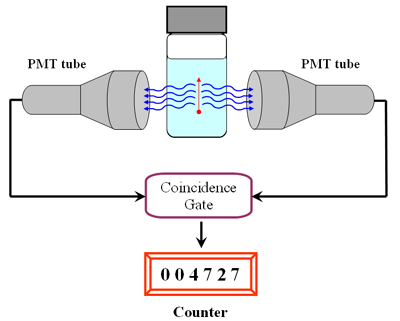
Photons are emitted from the scintillation vial in all directions at once. Two PMT tubes, located on opposite sides of the vial, act as light detectors. A coincidence circuit is set to discriminate between noise and pulses caused by β particles. Only in the latter case will light strike both detectors simultaneously, and the coincidence gate will announce that a beta decay has occurred.
The chance of noise pulses reaching both PMTs simultaneously is very small. If the coincidence circuit detects a pulse from one PMT and not from the other - within 40 ns, the analyzer will disregard the pulse. This coincidence circuit insures that the liquid scintillation counter's background is kept at about 20 - 40 cpm.