The Interdepartmental Equipment Facility
EDS - Energy Dispersive X-ray Spectroscopy

Elemental Analysis by EDS is achieved by monitoring and analyzing x-rays emitted by matter when bombarded with charged particles. Because of its unique atomic structure, each element radiates x-rays at characteristic wavelengths which allows its identification.
The Jeol JSM 5410 SEM in our Microscopy-Lab is equipped with an Oxford Link Isis - Energy Dispersive X-ray Spectrometer (EDS), which serves as an elemental analyzer. The solid state Si(Li) detector, permits the detection of X-rays and identification of the elements responsible for the emission in a microscopic location.
Principles of Operation.
Practically all atoms in the sample are in their ground state before they are hit by the incident electron beam of the SEM. The effect of the beam on a sample atom may be to eject an electron from an inner shell creating a hole. Another electron, from an higher-energy shell now drops into the hole, and the difference in energy between the shells is released as an X-ray photon.
The electron beam of the SEM induces different excited states in different atoms on the surface of the sample, and these excited states dissipate by emission of X-ray radiation at different wavelengths, each characteristic of the element involved.
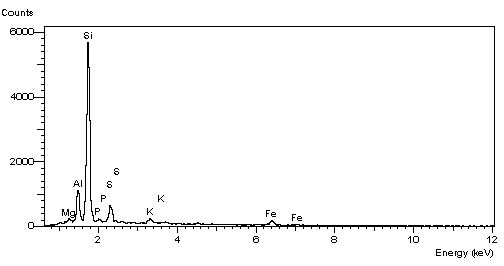
EDS facilitates qualitative and quantitative elemental analysis from boron (B) to uranium (U). The analyzer matches an element to its signature peaks, leading to its proper identification. By utilizing the scanning feature of the SEM, a spatial distribution of elements, can be obtained. Detection limits for different elements range between 0.1-1% (% by weight). Quantitative analysis can reach an accuracy of 1-3% for flat, polished homogeneous samples, when appropriate standards are available. A typical energy spectrum and the corresponding elemental analysis result is presented below.