The Interdepartmental Equipment Facility
Thermo Polaris Q - An Ion Trap GC/MS

GC/MS or Gas Chromatograph Mass Spectrometer, is used to separate, detect, identify and determine the content of chemicals in a large variety of samples. These could be contaminants in drinking water or air samples, residues of pesticides in agricultural produce or drugs in physiological fluids. We use it also to identify and determine the level of metabolites and natural products in extracts of plant or animal tissues. The Thermo Polaris Q is an ion-trap GC/MS Equipped with a Trace GC and a triplus autosampler. Its MSn-capabilities facilitate structure confirmation and elucidation.
Main Features and Specifications
- EI (Electron Impact) and CI (Chemical Ionization) modes.
- Exchangeable Ion Volumes.
- GC interface temperature up to 350oC.
- Mass range 10-1000 amu, with unit mass resolution.
- Scan rate of 5555 amu/sec.
- Automatic Gain Control.
- Offers full-scan, segmented scanning, SIM, and MSn (n=1 to 5) modes of mass analysis.
- Inlet / Vacuum Interlock Allows easy removal of ion volumes.
Sample Preparation.
Most samples have to be prepared for analysis. Solid Phase Extraction
and Liquid-Liquid Extraction are commonly used for liquid samples. The
efficiency of the process is estimated by measuring the "recovery" of
single compounds in the preparation and sample introduction procedures.
The sample is "spiked" with known amounts of the compound before
sample-preparation. Internal standard and surrogate standards are also
added to monitor these processes and evaluate their quantitative significance.
Chromatographic Separation.
The GC separates complex mixtures
of organic compounds on a chromatographic column. The MS detects, identifies
and measures the quantity of the separated compounds one by one, as they come
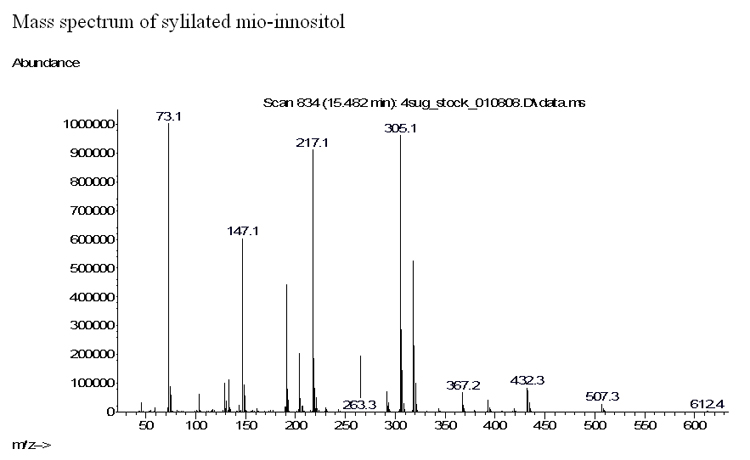
out of the column. The chromatogram shown below illustrates the separation
of a mixture of sugars by GC/MS on a time scale of about half an hour.

The Mass Spectrum. As each one of the separated compounds reaches the detector, it is trapped there for a short time in the 'ion-trap'. During this period the compound is bombarded by fast electrons which break the molecule into fragments and the Mass Spectrometer records the ratio of mass/charge for each of these fragments.
This collection of m/z data for the daughter-fragments is the 'mass-spectrum' of the parent compound that serves as a set of 'fingerprints'. The computer now searches for a match for this specific mass spectrum, through libraries containing hundreds of thousands of mass spectra, and tries to identify the parent compound.
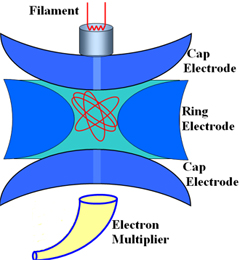
The Ion Trap Mass Spectrometer
The processes carried out by the Mass Spectrometer include several steps:
1. Ionization,
2. Fragmentation,
3. Mass Analysis,
4. Detection
Ionization, fragmentation and mass analysis, all take place inside the trap. Mass detection is carried out by the electron-multiplier located below the ion trap
The Ion-Trap The Ion Trap analyzer consists of three electrodes. The top and bottom electrodes ('the caps') are hemispherical. The center electrode is ring- or doughnut-shaped. The illustration on the right shows an ion trap cut in half. The end of the GC capillary column is directly connected to the Ion-Trap. The trap is filled, at fixed intervals, with sample fractions as they come off the GC column.
Ionization and Fragmentation Sample molecules have to
be ionized before they can be analyzed and detected by the MS. The
ionization methods most widely used in GC/MS work are:
1. EI - Electron Impact
2. CI - Chemical Ionization
EI - Electron Impact Ionization. In an EI source, sample molecules are bombarded by electrons. The electrons are generated by a heated filament and accelerated towards a positive target by a 70 V potential difference. When a speeding 70 eV electron strikes on its way a neutral sample molecule, it knocks out one of its electrons. Additional energy absorbed by the molecule induces vibrations and rotations and molecular rearrangements. The result is fragmentation of the molecule.
EI is usually described as a 'hard' ionization method. While it yields structural information via fragmentation, at times the fragmentation can be too extensive, so that the molecular ion can be difficult or impossible to find. This is especially true for larger molecules. Chemical ionization , on the other hand, which relies on transfer of charge from a reagent ion to a sample molecule is considered to be a soft ionization method: it results in reduced fragmentation and increased yield of the molecular ion.
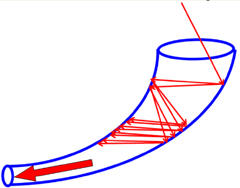
Mass Selection. Once the sample is ionized, the ions are trapped inside by the oppositely charged walls of the trap. Controlled attenuation of the voltages on the electrodes permits selected masses to exit the trap towards the detector where their quantity is measured.
The Ion detector most commonly used is the electron multiplier - a continuous dynode device. When the inner surface of the detector is struck by an ion, electrons are ejected. The ejected electrons are accelerated by a potential gradient down the device and as they hit the surface again and again, more and more electrons are ejected and increase the current. This electron multiplication process amplifies the electrical signal by many orders of magnitude.